Basic Information
Product Name: DLMR Premium
Function: Anti-aging, skin care, collagen stimulator
Volume: 2 ml / Vial Liquid
Shelflife: 3 Years
Storage: Store away from light and room temperature
Target People: Sagging skin lacks elasticity, collagen loss, and fine lines
Origin: South Korea

It is developed and produced by Dexlevo, a aesthetic medicine company specializing in the development of soluble biodegradable polymer technology.
Each ampoule contains 30% PCL (polycaprolactone), which is extremely pure. As a new type of filler, PCL has good biodegradability, and it is the main raw material for thread carving and medical absorbable suture.
Soluble PCL only needs to be applied on the face at 8~10 points, and it can naturally spread to all parts of the skin, which can reshape the facial structure, promote collagen, and has the effects of lifting and firming, restoring elasticity, and slowing down skin aging. The effect is more durable and stable compared with other lifting products on the market.
There are a lot of hyaluronic acid dermal filler in the market, however the duriation time is not long.
However, the product like BDDE or DVS cross linked hyaluronic acid particles and commonly used polymer products (polylactic acid and polycycloacetone) last longer but are not easy to degrade. And this microparticle product has the risk of vascular embolism, and it is not easy to spread in the skin, so it can only be used on some part of the face.
Dexlevo pioneered the CESABP patented technology (full name: Colengisation-International Solubilized Active and Biodletablection Polmer), and developed the easily biocompatible and biodegradable polymer PCL.
Main Function:
Full Layer Anti-aging
PCL can stimulate fibroblasts and promote the synthesis and regeneration of natural collagen. Eight-point boost will be a "new generation anti-aging" collagen stimulator, which can effectively deal with aging skin.
Firming
It directly promotes the regeneration of collagen molecules, reorganizes, repairs and tightens the loose superficial fascia fat cell chains, so as to achieve a lifting effect.
Enhance elasticity
Effectively promote the regeneration of subcutaneous collagen and elastin, restore the skin's elasticity, regain its youthful state, and make the skin firm, lifted, plump and youthful.
Anti wrinkle
After the biodegradable PCL enters the human body, the cortical filler forms an extensive 3D matrix, which can be completely absorbed by the human body, effectively promotes the regeneration of natural collagen, and continuously reduces fine lines and wrinkles.
Direction for Use
Suggested needle: 30~34G sharp needle (13mm)
Needle entry method: vertical needle entry
Suggested dosage: 0.2~0.5cc/per point
Recommended depth: 5~8mm deep dermis on the forehead; 8~10mm deep dermis on the cheeks.
Suggested course of treatment: 2 courses of treatment in 1 year, 2 times for each course of treatment, 1 bottle each time. The second dose was given one month later, and the course of treatment was repeated six months later.
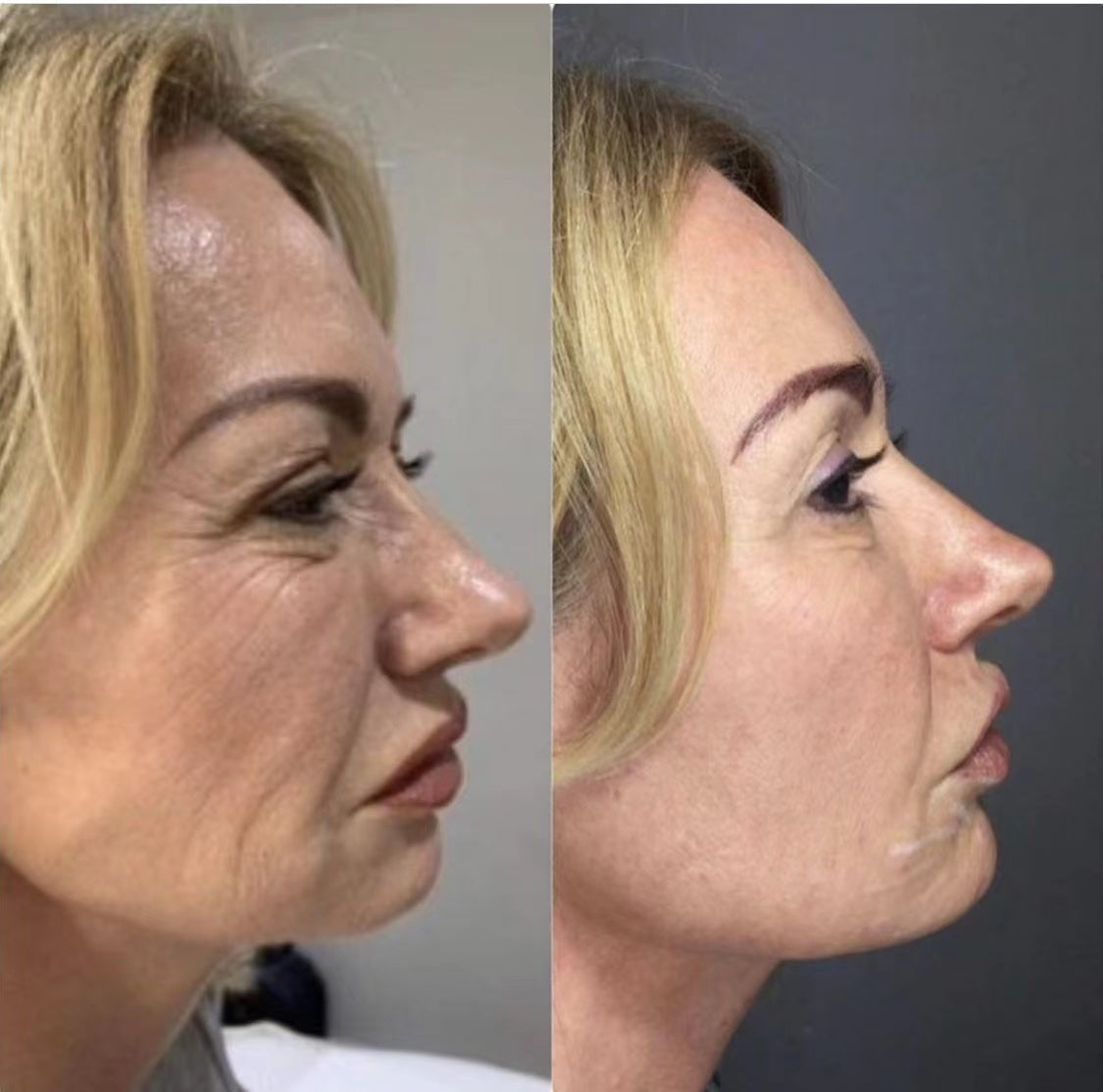
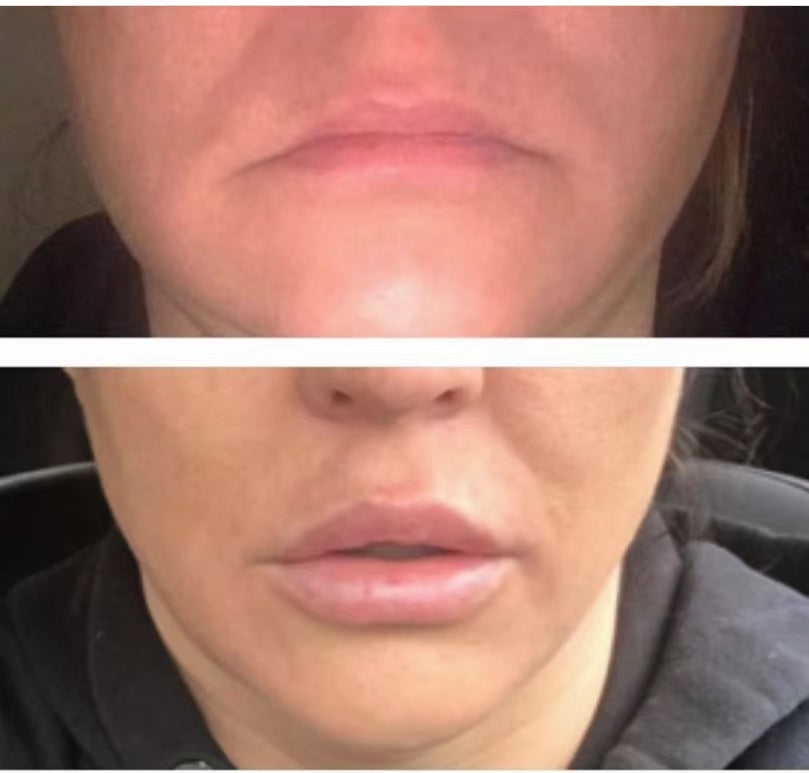
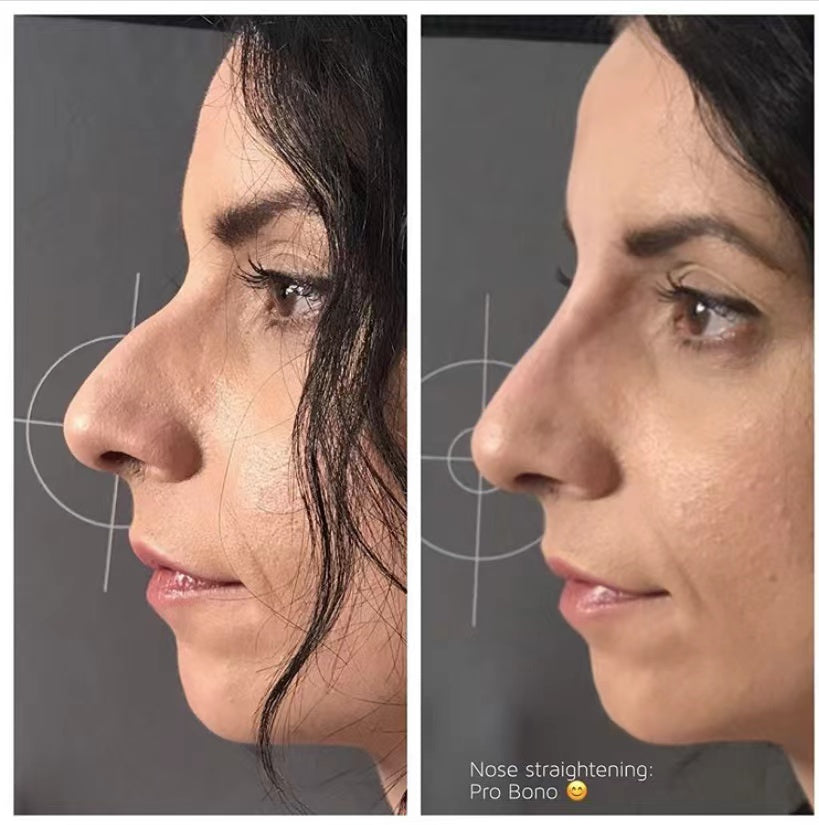
Before & After:


Experimental data:

No definite nodules were found after 8 h in the DLMR01 group. Throughout the 12-week testing period, dermatologists observed no overt signs of inflammatory erythema, swelling, and pus. Furthermore, delayed responses including nodule formation and granulomas were not detected in either group. Therefore, both PBS and DLMR01 were considered to be well tolerated.
To examine changes in dermal collagen content, MT staining was performed. The DLMR01-injected group showed a significant increase within the first 6 weeks. The dermal thickness of DLMR01-injected rats was significantly increased, especially in the first 6 weeks. Skin growth slowed after 12 weeks.
Biocompatibility Test Results
Patent No. (Korea): 10-2018-0058947
Patent Number (USA): 16/410,611
Patent number (PCT): PCT/KR2018/005988
Q&A
What is DLMR Dermal Lifting Filler?
"Dermal fillers have become popular due to the increased demand for skin rejuvenation products. Polycaprolactone (PCL), a newly developed bioresorbable medical polymer, has emerged as a durable and safe dermal filler. However, available PCL fillers cause irritation; carrier gels can coagulate PCL particles, block the injection needle, and cause nonhomogeneity of particle suspensions that could be responsible for the observed side effects. To relieve pain, premixing PCL filler with lidocaine. However, this formulation changes the property of the CMC portion of the PCL filler, and possibly results in an uneven suspension of the PCL particles. Hence, a particle-free PCL homogeneously solubilized in water was developed to overcome these limitations. This study aimed to assess the in vivo safety, biodegradability, and neocollagenesis ability of a novel PCL filler, DLMR01 using a rat model. Fillers were characterized after injecting a vehicle control or DLMR01 using a digital camera, folliscope, and a three-dimensional profiling system. Biopsy was performed to evaluate biocompatibility and neocollagenesis. Skin elasticity was measured using a Cutometer. DLMR01 caused no needle occlusion by particle aggregation or laborious injectability. Filler nodules dispersed to surrounding tissues within 6 hours without further granuloma formation. Histological inspection revealed no tissue residual material or foreign body reaction during the 12-week test period. DLMR01 increased dermal thickness, collagen regeneration, and skin elasticity. In conclusion, this study demonstrates the potential of DLMR01 for dermal rejuvenation in a rat model."
References:In vivo evaluation of novel particle-free polycaprolactone fillers for safety, degradation, and neocollagenesis in a rat model
Ji Yeon Hong 1, Jong Hwan Kim 2, Tae-Rin Kwon 2, Jun Ki Hong 2, Kapsok Li 2, Beom Joon Kim 2
Why PCL instead of PLLA?
Although both of them are orthopedic filling materials, which can induce collagen production, the metabolism of PLLA is relatively slow, and the lactic acid reaction will cause discomfort, so it is more suitable for orthopedic filling materials to induce bone regeneration.
On the contrary, low-molecular-weight PCL metabolizes very quickly, and the metabolites will not cause discomfort. However, due to the fast metabolism, it is not suitable for inducing bone regeneration. It is more suitable for medical aesthetic materials and becomes a better choice for skin regeneration. The exclusive CESABP patented technology has been implemented in various in vivo and in vitro biocompatibility tests, and the US NAMSA has evaluated it as completely biocompatible according to ISO standards.
We deliver fully soluble liquid PCL into the face through eight points, and it can spread to a wide range of skin without any side effects such as lumps or nodules, and the cortical filling forms a wide 3D matrix, which can be maintained for a long time, and effectively promote natural collagen regeneration, maintain facial skin healthy and elasticity. Improve facial aging problems effectively and safely.