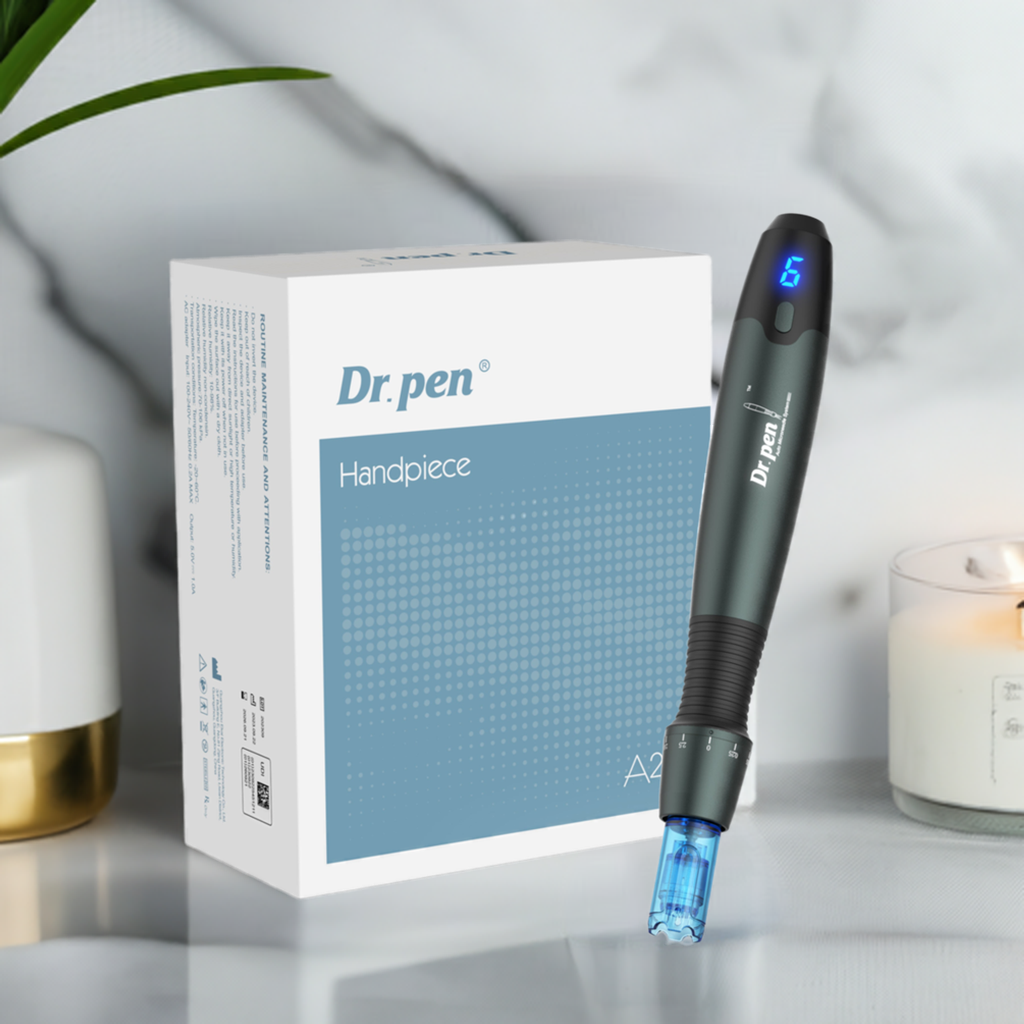
FDA 510K Cleared Dr. Pen A20 Microneedling Pen
Dr.Pen A20 Microneedling Systerm is FDA 510K Approved Class II Medical Device. 510K Number: K230420 FEI Number*: 3013360665As a cornerstone technology for modern aesthetic practices, the Dr. Pen A20 represents...




